Cost-Effectiveness of the Geriatrician-Led Comprehensive Geriatric Assessment in Different Healthcare Settings: An Economic Evaluation
ABSTRACT
Background
With a shortage of geriatricians, the appropriate distribution of geriatricians across healthcare settings (e.g., acute care, rehabilitation, or community clinics) is unknown. Our objective was to determine which setting(s) geriatricians should preferentially staff to be most economically attractive for the Canadian healthcare system.
Methods
We conducted a cost-effectiveness analysis using a two-dimensional microsimulation model. The model simulated a population of frail adults aged ≥ 65 years. The simulation was done over a lifetime horizon from the Ontario public payer perspective. Strategies included (1) usual care (baseline proportions of geriatrician CGAs in each setting), (2) acute care only (100% receive CGA in acute care), (3) community care only, (4) rehabilitation only, (5) acute care and community combined, (6) acute care and rehabilitation combined, (7) community and rehabilitation combined, and (8) acute care, community, and rehabilitation combined. Primary model outputs included quality-adjusted life months (QALMs), lifetime costs, and incremental cost-effectiveness ratios (ICERs).
Results
The acute care and rehabilitation combined strategy was undominated at a lifetime cost of C$139,987 and with an effectiveness of 42.09 QALM. At an ICER of C$1203 per QALM, the combination strategy of acute care, rehabilitation, and community clinics was cost-effective relative to acute care and rehabilitation, assuming a cost-effectiveness threshold of C$4167 per QALM (equivalent to C$50,000 per quality-adjusted life year). The other six strategies were dominated. When individually compared to usual care, all of the strategies were dominant or cost-effective.
Conclusions
An undominated strategy of staffing geriatricians was in the acute care and rehabilitation settings, with the option of adding community clinics if cost and resources permit.
Summary
-
Key points
- ○
The shortage of geriatricians in Canada can be addressed by optimizing their distribution across healthcare settings.
- ○
We used a cost-effectiveness analysis to determine what combination of healthcare settings is optimal for the staffing of geriatricians.
- ○
The combination of acute care and rehabilitation settings was undominated.
- ○
The addition of community clinics to acute care and rehabilitation settings was still cost-effective if resources permit.
- ○
-
Why does this paper matter?
- ○
The global shortage of geriatricians requires alternative solutions in addition to recruitment efforts.
- ○
Using an economic model, we demonstrated that staffing geriatricians preferentially in acute care and rehabilitation settings would provide the best cost-effectiveness if geriatricians could not be staffed in all settings.
- ○
1 Introduction
The comprehensive geriatric assessment (CGA) is a multidimensional approach to assessing and managing older patients with multimorbidity and frailty [1]. It can be completed in different settings (Table S1) including acute care (hospital-based inpatient care), community clinics (outpatient, ambulatory or in-home care), rehabilitation facilities (hospital-based rehabilitation care), and long-term care homes (LTC, residential care in a nursing home). Randomized trials in various settings have shown the effectiveness of CGAs in preventing nursing home admissions, functional decline, hospital readmissions, health resource utilization, and death [2, 3]. Previous economic analyses demonstrated the cost-effectiveness of the CGA in acute care settings [4, 5]. The CGA can be led by a geriatrician (specialist physician in geriatric medicine) or other clinicians (e.g., nurses [6], social workers [7], physiotherapists [8], or other physicians [6]). Preliminary results from a network meta-analysis showed the superior efficacy of a geriatrician-led CGA compared to a non-geriatrician-led CGA (unpublished [9]). However, the cost-effectiveness of a geriatrician-led CGA across different settings is unknown.
Similar to the United States, geriatricians in Canada receive training in internal medicine followed by 2 years of geriatric medicine subspecialty residency (some training programs in the United States are 1 year in length) [10]. Geriatricians have specific knowledge and skills pertaining to diseases of older adults, but there are few geriatricians in Canada (0.57 geriatricians for every 10,000 people aged ≥ 65 years) [11]. A similar shortage of geriatricians exists in the United States (1.36 geriatricians for every 10,000 people aged ≥ 65 years) [12]. Given this scarcity, it is of interest to determine the optimal deployment of the geriatrician workforce. If we can identify the healthcare settings where the geriatrician-led CGA is most economically attractive, we can optimize deployment or recruitment to more efficiently allocate scarce geriatrician resources. Priority setting is timely given the size of the ≥ 65 population in Canada and given that an estimated 10.7 million Canadians will be aged ≥ 65 years by 2040 [13].
A cost-effectiveness analysis is one way of comparing the economic attractiveness of different strategies or interventions (e.g., the geriatrician-led CGA in different healthcare settings). Using a model-based simulation, we can determine the effectiveness of the different strategies, which is outputted in quality-adjusted life years (QALYs) or quality-adjusted life months (QALMs). QALYs and QALMs incorporate both length and quality of life into one measure. The incremental cost-effectiveness ratio (ICER) represents the cost increase per QALY or QALM by adopting one strategy over another. Our objective was to determine the allocation of geriatricians across healthcare settings (acute care, community clinics, and rehabilitation) that is most economically attractive for the Ontario population from a public payer perspective using a model-based cost-effectiveness analysis.
2 Methods
We constructed a two-dimensional microsimulation model to compare lifetime costs and QALMs for geriatrician-led CGAs across different healthcare settings (acute care, community care, and rehabilitation). A two-dimensional model simulates individuals with a range (or distribution) of characteristics at the person and population levels. For example, characteristics such as age and sex are sampled (or varied) at the individual level, while efficacy parameters (e.g., LTC admission benefit of a geriatrician-led CGA) are sampled at the population level. By running the model across a range of individuals within a range of populations, we can determine the uncertainty of the model outcomes.
The study was conducted according to the Ontario public payer perspective because healthcare costs are mainly paid for by the provincial governments in Canada. Costs and QALMs were discounted at 1.5% per year based on Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines (now known as Canada's Drug Agency) [14]. Future costs and health benefits were discounted (meaning they are worth less) compared to today's costs and benefits to reflect interest rate effects and lost opportunity costs [14]. The model was constructed in TreeAge Pro 2024 (TreeAge Software Inc., Williamstown, MA). The results were reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [15]. The protocol was registered with Open Science Framework (https://osf.io/dnw6x/). Details of the simulation model and other methods are provided in Appendix S1. Definitions of health economic terms used in this paper are in Appendix S4 along with an overview of the process in conducting the analysis.
2.1 Simulated Patient Characteristics
We sampled characteristics (Table 1) of frail older adults from individual-level distributions including age, sex, cognitive scores (mini-mental status examination [MMSE] score), and functional scores (Katz activity of daily living [ADL] score). Not all older adults age ≥ 65 years require a CGA, so we used frailty as the indication for a CGA in this model, which is supported by prior literature [16].
| Characteristic | Value | Original cohort | Frailty definition |
|---|---|---|---|
| Age, mean (SD) | 82 (9.6) | Ontario administrative database | Validated database 2/7 criteria |
| Female sex, % | 60.2 | Ontario administrative database | Validated database 2/7 criteria |
| MMSE score, mean (SD) | 16.7 (6.1) | Canadian study of health and aging | Clinical frailty scale ≥ 5 |
| Katz ADL score, mean (SD) | 5.3 (0.9) | Community dwelling older adults in France | Fried frailty phenotype |
- Abbreviations: ADL, activities of daily living; MMSE, mini-mental status examination; SD, standard deviation.
2.2 Model Structure
Simulated individuals started in one of the healthcare settings according to the proportions of older adults within those settings in Ontario. Healthcare settings used in our simulation model (Appendix S2: Figure S1) included community dwelling, acute care, rehabilitation, and LTC. The model progressed in time steps or cycles, where the simulated individual can remain in the same setting or they can encounter a health event that leads to a transition in healthcare setting. For example, community-dwelling individuals can be admitted to acute care or LTC. Individuals in acute care can be discharged to the community, rehabilitation, or LTC. Individuals can also die in any of the settings. Each cycle lasted 1 month in the acute care and rehabilitation settings and 12 months in community dwelling and LTC settings, which reflected the health stability of individuals in the respective settings.
2.3 Strategies
Due to the lack of effectiveness data in the LTC setting (preliminary results from Soobiah et al. [9]), we included combinations of CGAs in the other settings as strategies: usual care, acute care only, community care only, rehabilitation only, acute care and community, acute care and rehabilitation, rehabilitation and community, or all three. Usual care is defined as the current level of geriatrician-led CGA in the community and hospital settings. Geriatrician-led CGAs was provided to 5.9% of frail adults age ≥ 65 years in the community and 4.3% of those in acute care hospital [17]. The proportion of geriatrician-led CGAs in the rehabilitation setting was assumed to be 2.2%. For the other strategies, we assumed that 100% of frail older adults in that setting received a geriatrician-led CGA.
2.4 Model Probabilities, Cost and Utilities
Efficacy parameters (Table 2) in the model were derived from a systematic review and network meta-analysis comparing the effectiveness of a geriatrician-led CGA versus usual care [9]. Event probabilities (e.g., admission to LTC or death) in the geriatric rehabilitation setting were derived from a systematic review of randomized trials of geriatric rehabilitation interventions in older adults age ≥ 65 years [26]. Other transition/event probabilities, costs, and utilities (Table 2) were derived from a MEDLINE literature search (from inception to September 2022; search keywords are in Table S3). Estimates from randomized controlled trials and systematic reviews were preferentially chosen, supplemented by non-randomized studies. Cost data were derived from the Ontario Ministry of Health and Long-Term Care (MOHLTC), the Canadian Institute for Health Information (CIHI), and expert consultation. Costs are in Canadian dollars (C$) and inflated to 2023 values using the Bank of Canada inflation calculator.
| Estimate, mean (SD) unless specified | Reference | |
|---|---|---|
| Efficacy estimates | ||
| Improvement of MMSE with CGA, community dwelling | 0.70 (0.643) | Soobiah (unpublished [9]) |
| Improvement of MMSE with CGA, hospitalized | 1.26 (0.462) | Soobiah (unpublished) |
| Odds ratio of admission to LTC with CGA, community | 0.97 (0.084) | Soobiah (unpublished) |
| Odds ratio of admission to LTC with CGA, hospitalized | 0.80 (0.059) | Soobiah (unpublished) |
| Odds ratio of admission to LTC with CGA, rehab | 0.68 (0.247) | Soobiah (unpublished) |
| Odds ratio of mortality with CGA, community | 0.96 (0.071) | Soobiah (unpublished) |
| Odds ratio of mortality with CGA, hospitalized | 0.92 (0.043) | Soobiah (unpublished) |
| Odds ratio of mortality with CGA, rehab | 0.81 (0.133) | Soobiah (unpublished) |
| Change in ADL score with CGA, community |
−0.03 (0.152) |
Soobiah (unpublished) |
| Change in ADL score with CGA, hospitalized | 0.14 (0.140) | Soobiah (unpublished) |
| Change in ADL score with CGA, rehab | 0.16 (0.416) | Soobiah (unpublished) |
| Probabilities in community | ||
| Dementia | Table based on age, sex, and risk increase based on MMSE score | [18, 19] |
| LTC admission | Derived from table of LTC admission by age | [20] |
| Death | Life tables multiplied by relative risk of dementia | [21] |
| Relative risk of death with dementia | 2.7 (0.3) | [22] |
| Probabilities in acute care | ||
| Cognitive decline | 0.246 (0.0045) | [23] |
| LTC admission | 0.04 (0.0133) | [24] |
| Death | 0.04 (0.0133) | [24] |
| Rehabilitation transfer | 0.02 (0.0067) | [24] |
| Length of acute care stay | 8.9 days* | [25] |
| Probabilities in rehabilitation | ||
| Functional recovery | 0.159 (0.108) | [26] |
| Hospitalization | 0.047 (0.009) | [26] |
| LTC admission | 0.107 (0.022) | [26] |
| Death | 0.035 (0.013) | [26] |
| Length of rehab stay | 23.4 days* | [26] |
| Probabilities in LTC | ||
| Death, per year | 0.199 (0.066) | [27] |
| Hospitalization, per year | 0.257 (0.086) | [27] |
| Costs (in Canadian dollars) | ||
| CGA, per consult | 401.30 (fixed) | [28] |
| Community dwelling, dementia, monthly | 1370.86 (456.96) | [29] |
| Community dwelling, independent, monthly |
Table (for drugs only) Age 65–74: 1642.00 Age 75–84: 2206.94 Age 85+: 2660.12 |
[30] |
| Community dwelling, dependent, monthly | 1665.36 (555.12) | [31] |
| Acute hospital, per day | 1112.88 (fixed) | [32] |
| Rehab stay, per day | 765.17 (fixed) | [33] |
| Long term care stay, monthly | 4996.09 (fixed) | [31] (MOHLTC pay for LTC is fixed, the rest is paid by co-pay or other sources of income by resident). |
| Utilities | ||
| Community dwelling, older adult | 0.76 (0.114) | [34] |
| Acute hospitalization, older adult | 0.7 (0.29) | [35] |
| Long-term care, older adult | 0.48 (0.08) | [36] |
| Disutility of dementia | −0.32 | [37] |
- Abbreviations: ADL, activities of daily living; CGA, comprehensive geriatric assessment; LTC, long-term care; MMSE, mini-mental status examination; MOHLTC, Ministry of Health and Long-Term Care (public healthcare payer); SD, standard deviation.
- * No standard deviation for the Poisson distribution for length of stay.
2.5 Cost-Effectiveness Analysis and Outcomes
The primary outcomes were discounted lifetime cost and discounted QALMs for the eight strategies. A strategy is dominant if it increases health benefit (QALMs) while decreasing cost (hence economically attractive). Conversely, a strategy is dominated if it decreases QALMs while increasing cost (hence not economically attractive). If a strategy increases QALMs with increased cost, then we need to determine whether the amount of health benefit is worth the cost, which is done using the ICER. We computed ICERs only between pairs of non-dominated strategies. We compared ICERs to a cost-effectiveness threshold of C$4167 per QALM (equivalent to C$50,000 per QALY, below which health interventions are generally viewed as cost-effective) [38]. Secondary outcomes included undiscounted life years (without adjusting for quality of life), final cognitive score (measured by MMSE score), final ADL score (measured by Katz ADL score), dementia prevalence, and LTC admission. The 95% credible intervals were reported for the primary and secondary outcomes, which reflect uncertainty in the estimates.
2.6 Model Validation
The model was internally validated by examining the face validity of the structure and by verification of internal estimates. Face validity was established by reviewing the model structure with a content expert (SS) and methods experts (WI and DN). Internal calculations were verified by examining averages of tracking variables (e.g., age, sex, dementia status).
External validation was performed by comparing outputs from the model (usual care strategy) with literature estimates obtained from sources that were not used in the model construction. Key outputs chosen for validation included undiscounted life expectancy, proportion with dementia, and proportion in LTC.
2.7 Scenario Analysis
In the main analysis, we assumed that 100% of eligible patients would receive a geriatrician-led CGA. However, policymakers may not be able to provide CGAs to 100% of eligible individuals due to resource limitations. We explored various scenarios (scenario analysis) by running the model with smaller proportions of individuals receiving a geriatrician-led CGA (5%, 10%, 25%, 50%, and 75%).
2.8 Value of Information Analysis
Value of information analysis estimates the opportunity cost of adopting the most cost-effective strategy overall despite the uncertainty in the model output (i.e., policy decisions made with imperfect information). Specifically, the difference in the economic value of a policy decision made with current imperfect information compared with a decision made with no model uncertainty is the expected value of perfect information (per person value). This expected value can be multiplied by the number of individuals affected by this decision to determine the population-level expected value of perfect information. Further research is worthwhile if the cost to conduct the research is lower than the population expected value. We further identified the model parameters contributing the most to opportunity cost by estimating the expected value of partial perfect information (EVPPI) for each population-level input parameter. Details are provided in Appendix S1.
3 Results
3.1 Sample Size and Validation
The final model used 25,000 first-order (individual-level sampling) and 20,000 second-order (population-level sampling) iterations (Appendix S2: Figure S2). For face validity of the model, the types of health transitions modeled closely resemble common health outcomes of frail older patients [39]. For external validation, the life expectancy in our model of the usual care strategy was 5.73 years, which is similar to 6.0 years in an external cohort of frail older adults in the United States (adjusted for age at inception and sex ratio) [40]. For external validation of LTC admission, the final proportion in the model was 19.3% in the usual care group. This is similar to 17.5% in adults aged ≥ 80 years in Australia in 2021, another publicly funded healthcare system [41]. The final proportion with dementia in our cohort was 0.56 in the usual care strategy, which is similar to the prevalence of 0.52 in a cohort of older adults with frailty from Finland [42].
3.2 QALM, Cost, and ICER
The least expensive strategy was obtained from the provision of geriatrician-led CGAs in acute care and rehabilitation settings (Table 3 and Appendix S2: Figure S3). The only other undominated strategy was the provision of CGAs in acute care, rehabilitation, and community settings. Relative to acute care and rehabilitation, the provision of CGAs in acute care, rehabilitation, and community settings resulted in 1.07 additional QALMs at an incremental cost of C$1275, yielding an ICER of C$1203 per additional QALM, which is below a cost-effectiveness threshold of C$4167 per additional QALM and is therefore cost-effective. The incremental cost-effectiveness plot shows the difference in cost (y-axis) vs. the difference in QALMs (x-axis) of model iterations between two strategies. The incremental cost-effectiveness plot for geriatrician-led CGAs in acute care, rehabilitation, and community settings vs. in only acute care and rehabilitation settings is shown in Appendix S2: Figure S4. All but one model iteration fell below the cost-effectiveness threshold line (dotted line), indicating high certainty in the cost-effectiveness of the triple-setting strategy.
| Strategy | Cost (C$) | ∆ Cost | Effect (QALM) | ∆ Effect | ICER | Dominance |
|---|---|---|---|---|---|---|
| Acute care and rehab | 139,987 (139,662–140,312) | 42.09 (42.08–42.10) | Undominated | |||
| Acute care only | 140,026 (139,700–140,351) | 38 (38–39) | 42.09 (42.08–42.10) | −0.00 | — | Abs. dominated |
| Rehab only | 141,077 (140,754–141,400) | 1090 (1088–1092) | 41.56 (41.55–41.57) | −0.53 (−0.53 to −0.53 | — | Abs. dominated |
| Usual care | 141,114 (140,791–141,437) | 1127 (1125–1129) | 41.56 (41.55–41.57) | −0.53 (−0.53 to −0.53 | — | Abs. dominated |
| Acute care, rehab, and community | 141,262 (140,935–141,590) | 1275 (1273–1278) | 43.16 (43.15–43.17) | 1.07 (1.07–1.07) | 1203 (1190–1277) | Cost-effective |
| Acute care and community | 141,319 (140,992–141,647) | 57 (57–57) | 43.14 (43.13–43.15) | −0.02 (−0.02 to −0.02) | — | Abs. dominated |
| Community and rehab | 142,114 (141,788–142,439) | 852 (849–853) | 42.61 (42.60–42.62) | −0.55 (−0.55 to −0.55) | — | Abs. dominated |
| Community only | 142,175 (141,850–142,501) | 913 (911–915) | 42.60 (42.59–42.61) | −0.56 (−0.56 to −0.56) | — | Abs. dominated |
- Abbreviations: Abs. dominated, absolute dominance; ICER, incremental cost-effectiveness ratio; QALM, quality adjusted life month.
The cost-effectiveness acceptability curve shows the probabilities where each strategy was most cost-effective (y-axis) over a range of cost-effectiveness thresholds (x-axis), which reflects uncertainty in the ranking of strategies. Our cost-effectiveness acceptability curve (Figure 1) shows that the acute care and rehabilitation strategy has the highest probability of being the optimal strategy at cost-effectiveness thresholds less than C$1203, and the acute care, rehabilitation, and community strategy has the highest probability of being optimal for cost-effectiveness thresholds above C$1203. The transition of optimality between the two highest ranked strategies is better visualized with the cost-effectiveness acceptability frontier (Appendix S2: Figure S5), where a dark line highlights the optimal strategy through the range of cost-effectiveness thresholds. The point where the optimal strategy changes is the ICER of C$1203.
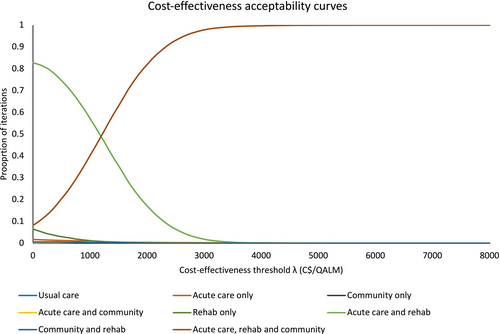
An alternative ranking table comparing all strategies with usual care is provided in Appendix S3: Table S5. When compared with usual care (current levels of CGA provision in each setting), all of the other strategies were more economically attractive. The strategies of acute care and rehabilitation, acute care only, and rehabilitation only were dominant over usual care. The remaining strategies were cost-effective when compared with usual care, assuming a cost-effectiveness threshold of C$4167 per QALM.
3.3 Secondary Outcomes
The combination of acute care, rehabilitation, and community clinic had the largest number of life years at 5.82 (95% CrI 5.82–5.82), which was the same as the combination of acute care and community (Appendix S3: Table S6). The combination of acute care, rehabilitation, and community clinic also had the lowest proportion in LTC at the end of the simulation (0.175, 95% CrI 0.175–0.175). The final MMSE score was also highest in the acute care, rehabilitation, and community clinics strategy (14.7, 95% CrI 14.7–14.7), which corresponds with the lowest prevalence of dementia (0.52, 95% CrI 0.52–0.52). The final ADL score was highest in the acute care and rehabilitation strategy at 3.66 (95% CrI 3.65–3.67), which was the same as the acute care only strategy. Overall, the combination of acute care, rehabilitation, and community clinic strategy had the best outcomes for life years, LTC admission, and cognition, while the acute care and rehabilitation strategy had a higher functional status (ADL) score.
3.4 Scenario Analysis
In the main analysis, we assumed that 100% of the cohort received a geriatrician-led CGA in the intervention settings (i.e., 100% of the cohort received a geriatrician-led CGA in the acute care setting for the acute care only strategy). When we lowered the proportion of the cohort receiving a geriatrician-led CGA in each strategy (Table 4), the acute care and rehab strategy was optimal when the proportion of CGAs ranged from 10% to 75%. The acute care, rehabilitation, and community clinic strategy was optimal when 5% or 100% of the cohort received a geriatrician-led CGA. In a scenario where only two settings could be staffed by geriatricians, the acute care and rehabilitation strategy was optimal when the proportion receiving the CGA ranged from 10% to 100%. When only a single setting could be staffed by geriatricians, the acute care only strategy was optimal when the proportion of CGAs ranged from 10% to 100%. Although the 5% coverage scenario suggested that community clinics were the optimal setting if a single setting could be staffed, there were no significant differences in the different strategies at that level of patient coverage (Appendix S3: Table S7). The ranking of strategies for each proportion of CGAs is shown in Appendix S3: Tables S7–S12.
| Percentage of frail older adults receiving geriatrician-led CGA in each setting | Optimal strategy (highest NMB) | ||
|---|---|---|---|
| Single settinga | Combination of two settingsb | All strategiesc | |
| 5% | Community only | Acute care and community | Acute care, rehab, and community |
| 10% | Acute care only | Acute care and rehab | Acute care and rehab |
| 25% | Acute care only | Acute care and rehab | Acute care and rehab |
| 50% | Acute care only | Acute care and rehab | Acute care and rehab |
| 75% | Acute care only | Acute care and rehab | Acute care and rehab |
| 100% | Acute care only | Acute care and rehab | Acute care, rehab, and community |
- a Single settings include: acute care only, rehab only, community only.
- b Two-setting combinations include: acute care and rehab, acute care and community, and rehab and community.
- c All strategies include: acute care only, rehab only, community only, acute care and rehab, acute care and community, rehab and community, and acute care, rehab, and community.
3.5 Value of Information Analysis
The expected value of perfect information for the decision to deploy geriatrician-led CGAs in acute care, rehabilitation, and community settings vs. just in acute care and rehabilitation settings was maximal (C$353 per person) when the cost-effectiveness threshold was equal to the observed ICER of C$1203 per additional QALM or C$14,434 per additional QALY (Appendix S2: Figure S6). Further research to refine the model parameters is worthwhile if it could be done for less than C$353 multiplied by the size of the population age ≥ 65 in Ontario. The two model input parameters with the highest per person EVPPI included the cost of being at home with functional dependence (C$74) and the cost of dementia (C$58) (Appendix S2: Figure S7). These are the two model parameters contributing most to the opportunity cost of residual uncertainty.
4 Discussion
In this economic evaluation, the undominated strategies were (i) staffing geriatricians in acute care and rehabilitation settings and (ii) staffing geriatricians in acute care, rehabilitation, and community settings. Further, we estimate that staffing in all three settings would be cost-effective relative to staffing geriatricians only in acute care and rehabilitation settings for the province of Ontario if sufficient geriatricians were available. This main analysis assumed that 100% of eligible frail patients ≥ 65 years could receive a geriatrician-led CGA in each setting. When compared to usual care, all of the staffing strategies were either dominant or cost-effective, suggesting that any of the staffing strategies are better than what is done currently.
In the scenario analysis, we varied the proportion of the cohort of older adults with frailty receiving a geriatrician-led CGA (Table 4). Since there are not enough geriatricians currently, policymakers can see which settings to staff preferentially at each level of patient coverage (from 5% to 100%). Currently, geriatricians see approximately 5.9% of patients in community clinics, 4.3% of patients in acute care, and 0.01% in long-term care [17]. For example, if there were a new hospital and there was only a single geriatrician, our analysis suggests that the geriatrician should preferentially staff the acute care inpatient setting for the greatest benefit. If the new facility included a rehabilitation unit, then that should be the next setting staffed by geriatricians. Other settings, such as outpatient clinics, could be supported by non-geriatricians conducting CGAs (e.g., other physicians, nurse practitioners, nurse specialists, occupational therapists, etc.).
There are several reasons why the acute care setting may have greater benefits when staffed by a geriatrician. First, hospitalization in older adults commonly leads to adverse outcomes, such as functional decline [43], cognitive decline [23], delirium [23], deconditioning [44], institutionalization, and death [24]. Being in acute care offers a strategic time of intervention for a geriatrician to prevent some of these outcomes. Second, hospitalized older adults are often complex, with a mix of acute medical or surgical illness superimposed on background multimorbidity. Familiarity with acute medical management favors the skill set of geriatricians, who have 3 years of internal medicine specialty training. Third, geriatricians are often tasked with administrative and leadership roles because of their teamwork and collaborative skills [45]. Working in an acute care setting allows geriatrician administrators to implement senior-friendly policies across an institution [46].
Staffing geriatricians in the rehabilitation setting would extend the benefits from an acute care stay, which is what our model shows (combination of acute care and rehabilitation being an undominated strategy). Geriatric rehabilitation has significant benefits in reducing mortality, LTC admission, preserving function, and improving cognition when compared to usual care [26]. Yet, geriatric rehabilitation is underutilized [47]. Providing geriatrician-led CGAs in the rehabilitation setting can address complex medical issues and polypharmacy and help with preserving function. There is no data on how many geriatrician-led CGAs are currently being done in the rehabilitation setting in Canada. Understanding how to optimally use geriatrician-led CGAs in the rehabilitation setting should be an area of research priority.
Assuming that 100% of frail older adults get a geriatrician-led CGA, the model shows that the combination strategy of all three settings is cost-effective relative to staffing in only acute care and rehabilitation settings. Ideally, if geriatricians can see all of the eligible patients, then they should work in all of the settings. The primary barrier to achieving this goal is the shortage of geriatricians in Canada [11]. It follows that Canada should develop a strategy to increase the number of geriatricians for its aging population. This recommendation is endorsed by the National Seniors Strategy [48]. A similar effort to increase the number of geriatricians in the United States has been championed by the American Geriatrics Society [12]. A limitation of the economic evaluation is that we did not account for the cost of training a geriatrician. However, the number of training spots is not the main barrier, as 19 out of 33 training spots were unfilled in the Canadian Medicine Subspecialty Match for 2024 entry [49]. In the United States, 234 of the 411 geriatric medicine positions were unfilled in the 2024 match [50]. Lack of interest from trainees is a barrier that educators, undergraduate medical programs, and policymakers need to address [51]. Low financial compensation is also a barrier to increasing the number of geriatricians [51, 52]. Geriatricians in the United States are paid, on average, US$20,000 per year less than internists, despite requiring 1 or 2 more years of training [53]. Since medical graduates in the United States have an average education debt of US$234,597 [54], geriatricians may need to receive higher financial compensation than internists to attract more trainees into the specialty.
There are some limitations of this cost-effectiveness analysis. First, the model assumed that repeat CGAs will offer the same benefit as the initial assessment. There is no existing literature on the effectiveness of repeat consultations. However, the prevalence of geriatric syndromes (e.g., dementia, falls, incontinence, polypharmacy, etc.) increases with age [55], so there may be additional benefits from repeat consults over time. Second, data were not available for the number of geriatrician-led CGAs in the rehabilitation setting, so the estimate was imputed by the average of the acute care and LTC proportions. Third, we did not explore the training and time-on-task requirements for non-geriatricians conducting a CGA, which may be explored in future studies [56].
5 Conclusion
The two undominated strategies of geriatrician staffing included (i) acute care and rehabilitation settings and (ii) the combination of acute care, rehabilitation, and community clinics. If enough geriatricians were available, staffing them in all three settings would be cost-effective for the province of Ontario.
Author Contributions
Conception or design: E.K.C.W., A.C.T., W.I., J.E.M.S., D.M.J.N., S.E.S. Acquisition, analysis, or interpretation of data: E.K.C.W., A.C.T., W.I., J.E.M.S., D.M.J.N., S.E.S. Drafted the manuscript or substantively revised it: E.K.C.W., A.C.T., W.I., J.E.M.S., D.M.J.N., S.E.S.
Acknowledgments
We thank Dr. Jennifer Watt for reviewing our abstract.
Conflicts of Interest
Eric Wong is supported by a Vanier Scholarship (Canadian Institutes of Health Research) and the Clinician Scientist Training Program at the University of Toronto. Dr. Straus is supported by a Tier 1 Canada Research Chair. Dr. Tricco is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis for Knowledge Users.
Linked Articles
This publication is linked to a related editorial by Avelino-Silva and Lee. To view this article, visit https://doi-org.bibliotheek.ehb.be/10.1111/jgs.19504.